Academy Of Pharmaceutical Affairs
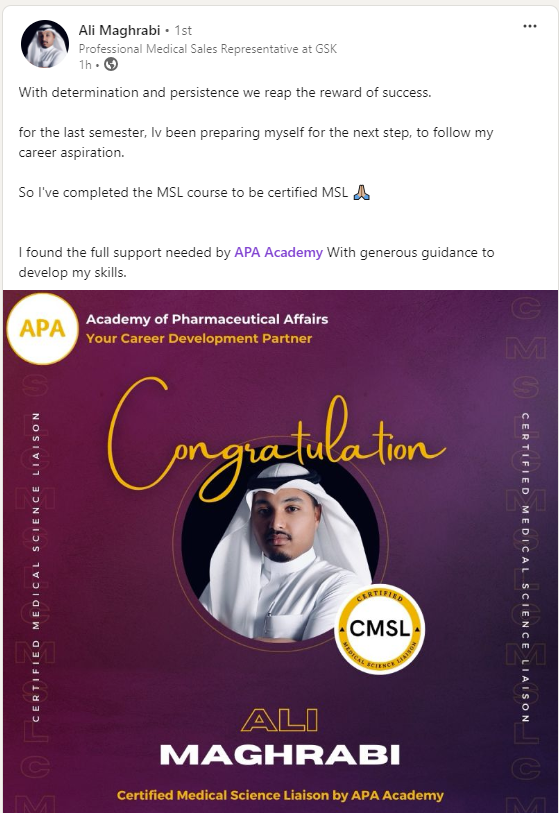
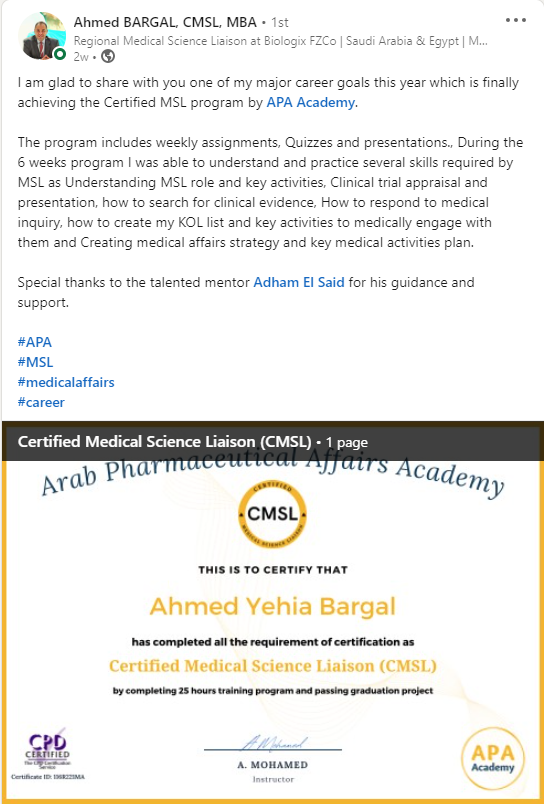
Certified Medical Science Liaison
Designed for Product Specialists, Medical Representatives and Aspiring MSLs.

CMSL Certificate equips you with the MSL competencies, practical skills, and strategic insights you need to excel as a Medical Science Liaison.
10 Interactive Live Sessions: (Learn)
Master clinical trial appraisal and presentation, medical strategy development, KOL engagement, and effective medical communication.
3 individual MSL assessments: (Apply)
Receive personalized coaching, comprehensive assessments, and tailored guidance that extends beyond certification, ensuring impactful career progression.
6 Month Mentorship Sessions: (Grow)
Enhance your professional growth with six dedicated sessions on business communication and interviewing skills, specifically designed to prepare you for successful MSL assessments.
Cohort 26
Starting April 25th, 2025
Start Your Learning Journey Now
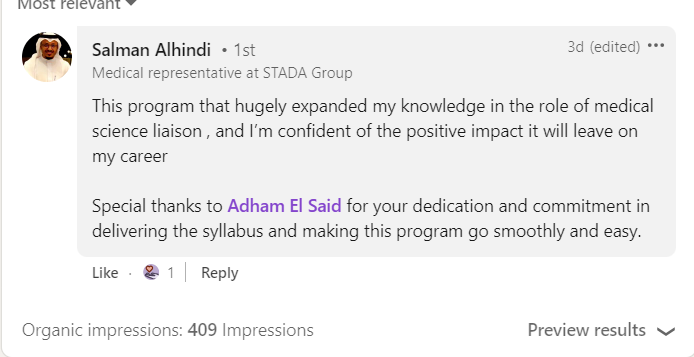
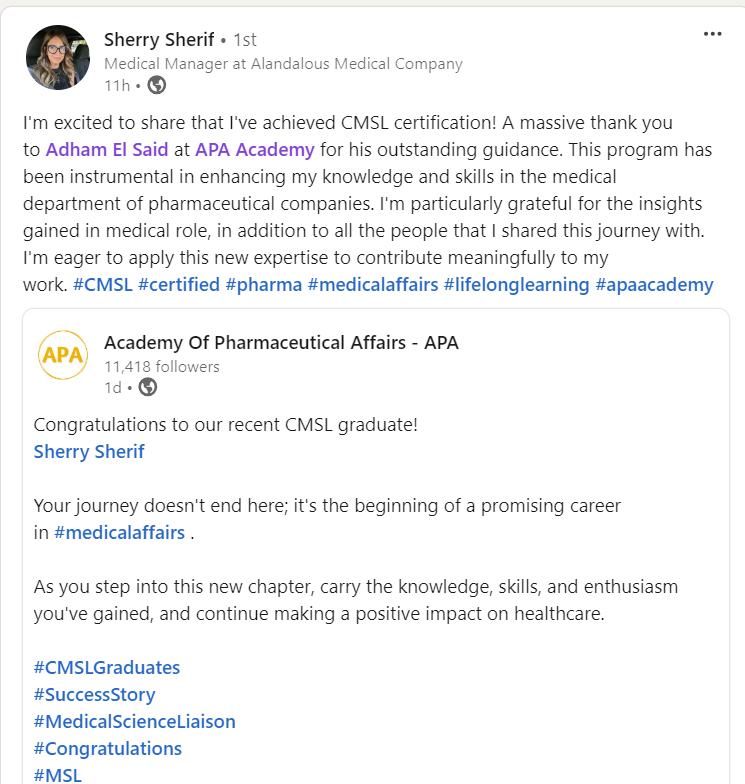
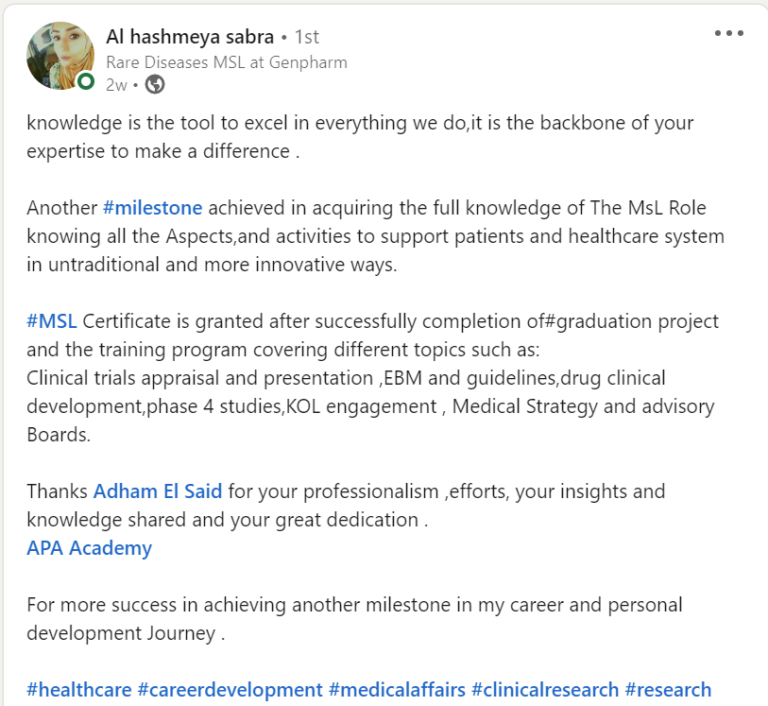
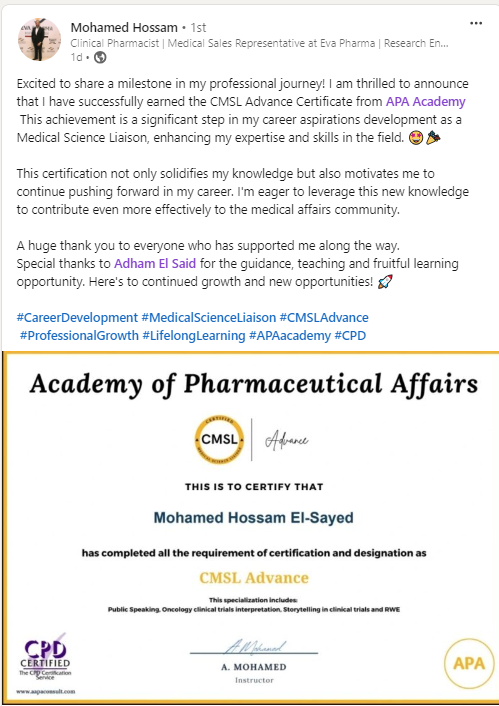
Join a community of more than 600 Graduate in the MENA Region in a Comprehensive Learning Journey Towards Medical Affairs













Your learning journey is extended beyond the course duration
Be ready for MSL assessment
10 online live Sessions. (4 hours/session) (Microsoft Teams)
Weekly Modules, with assignments, quizzes and final certification exam
3 Individual MSL assessments with feedback on Medical strategy and Clinical trials appraisal
After Certification support
Individual coaching and mentorship after graduation, including MSL assessment support
Program Instructor
Dr. Adham El Said
- 10 years Experience in a Regional Medical Affairs role (Middle East).
- 5 years experience in Medical Affairs capabilities training, trained more than 600 current and aspiring MSLs
- 15 years experience in the Pharmaceutical field across Middle East.
- Professional certified trainer from American University in Cairo.
- Qualified Professional trainer from ATD.
- Medical Affairs and soft skills trainer.
CMSL Learning outcomes
By the end of the program you will be able to:
- Understand the Role of a Medical Science Liaison (MSL) and the Required Competencies:
- Define the role of an MSL and explain its significance in the pharmaceutical industry.
- Identify the essential competencies and skills needed to be an effective MSL.
- Analyze the responsibilities and expectations of an MSL in different contexts.
- Answer Common Interview Questions:
- Recognize common interview questions typically asked in MSL job interviews.
- Apply effective strategies to answer interview questions confidently and convincingly.
- Evaluate and refine personal responses to common interview questions based on feedback and practice.
- Critically Appraise Clinical Trials:
- Analyze the components and design of clinical trials.
- Evaluate the quality and validity of clinical trial data.
- Synthesize and present a critical appraisal of a clinical trial.
- Create a Medical Strategy and Implement Different Medical Activities During the Product Life Cycle:
- Develop a comprehensive medical strategy for a pharmaceutical product.
- Plan and execute various medical activities aligned with the product life cycle.
- Justify the selection of specific medical activities based on the product’s stage and objectives.
- Create a Key Opinion Leader (KOL) List and Effectively Engage with Them:
- Identify criteria for selecting appropriate KOLs in a specific therapeutic area.
- Compile a KOL list based on the identified criteria and available resources.
- Develop strategies to engage and cultivate relationships with KOLs effectively.
- Understand Clinical Trial Terminologies:
- Define and explain key terminologies commonly used in clinical trials.
- Apply clinical trial terminologies to interpret and discuss trial protocols and results.
- Demonstrate proficiency in using clinical trial terminologies in written and oral communication.
- Professionally Present Each Section of a Clinical Trial:
- Demonstrate effective presentation skills in delivering each section of a clinical trial.
- Organize and structure information to ensure clarity and coherence in the presentation.
- Incorporate visual aids and supporting materials to enhance the understanding and engagement of the audience.
- Interpret Guidelines and Respond to Medical Inquiries:
- Interpret and analyze medical guidelines relevant to the pharmaceutical industry.
- Formulate accurate and comprehensive responses to medical inquiries based on guidelines and scientific evidence.
- Apply critical thinking skills to address complex medical inquiries effectively.
- Understand Different Types and Objectives of Post-Marketing Studies:
- Differentiate between various types of post-marketing studies.
- Identify the objectives and purposes of conducting post-marketing studies.
- Evaluate the relevance and value of post-marketing studies in different contexts.
- Manage Advisory Boards and Overcome Key Challenges:
- Demonstrate effective strategies for managing advisory boards in the pharmaceutical industry.
- Identify common challenges faced when working with advisory boards and propose solutions.
- Evaluate the outcomes and effectiveness of advisory board activities.
- Understand the Role of an MSL in Risk Management and Safety Education to Healthcare Professionals (HCPs):
- Explain the role of an MSL in risk management and safety education related to pharmaceutical products.
- Analyze the importance of safety education for healthcare professionals and its impact on patient outcomes.
- Develop strategies to effectively communicate risk management information to HCPs.
Our Graduates from more than 50 company across MENA Region





